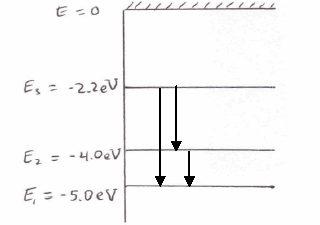
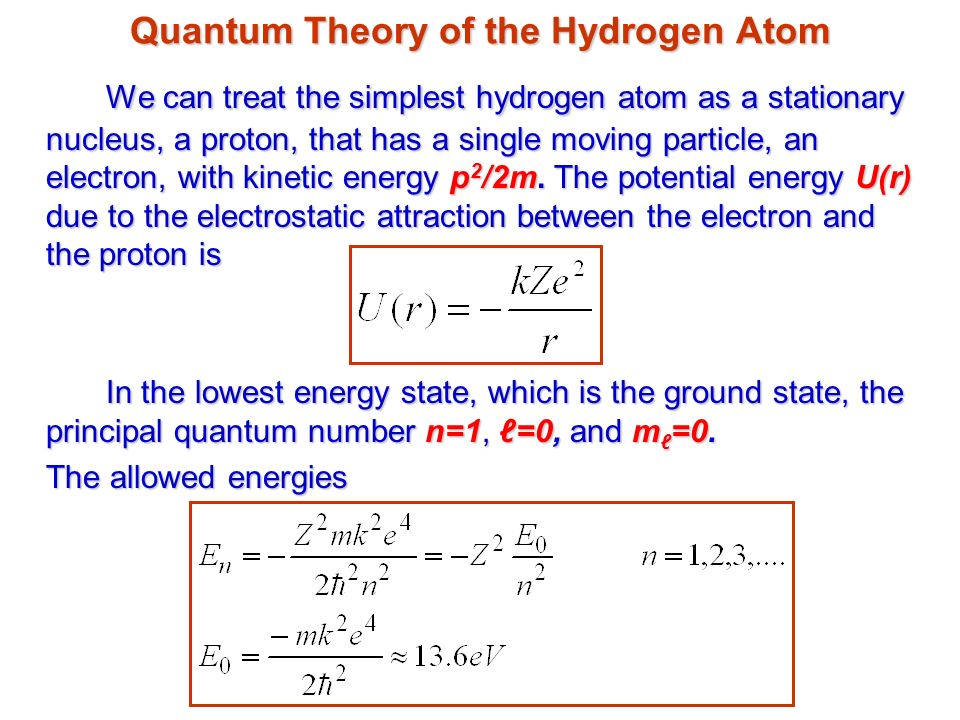
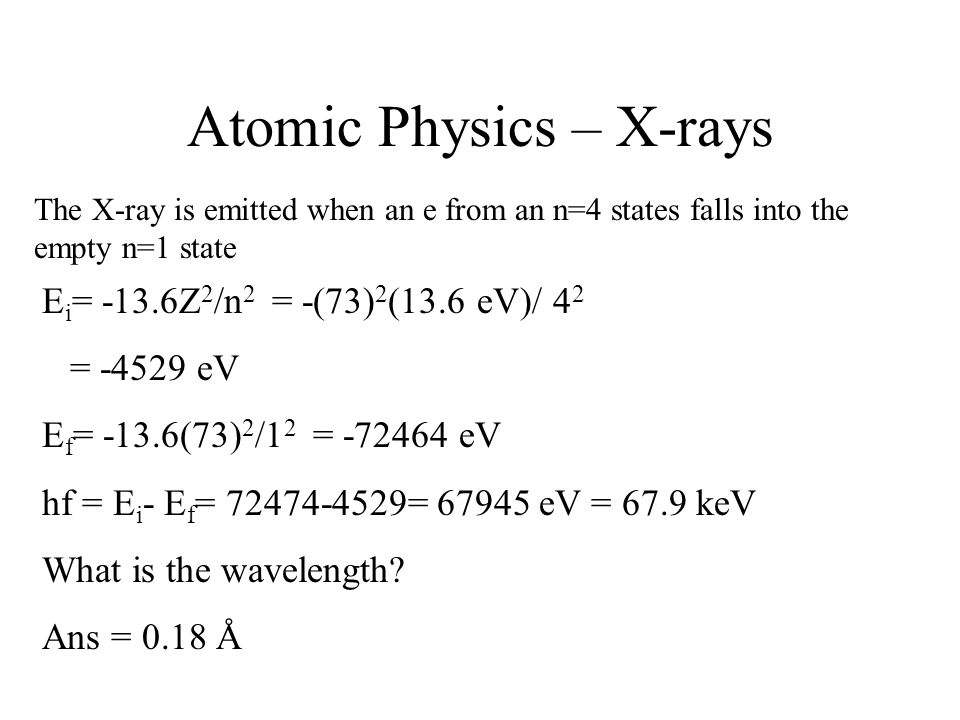
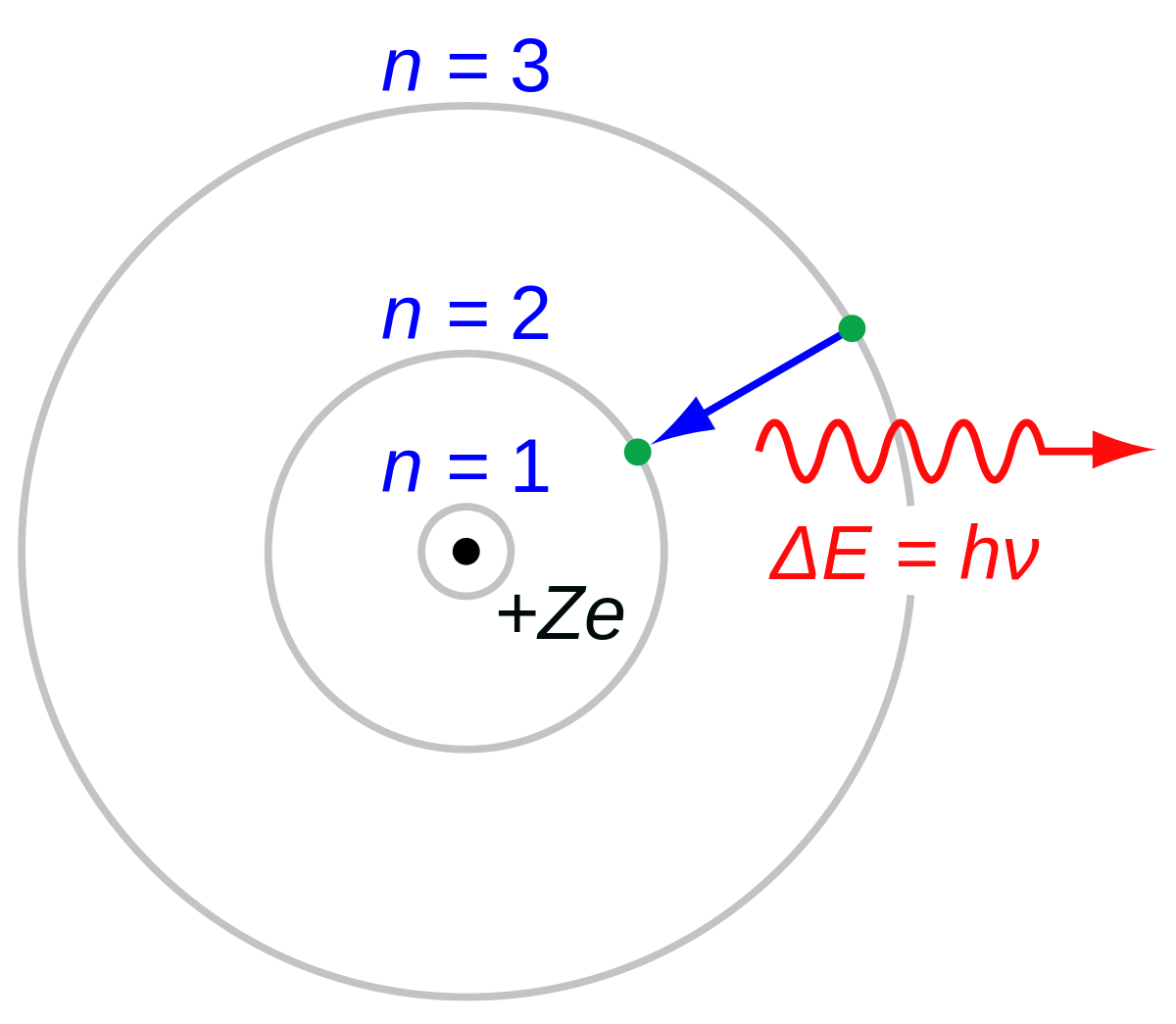
In a hypothetical Bohr hydrogen atom, the mass of the electron is doubled. The energy E0 and the radius r0 of the first orbit will be ( r0 is the Bohr radius)

CHAPTER 5 Atomic Models Much of the luminous matter in the Universe is hydrogen. In fact hydrogen is the most abundance atom in the Universe. The colours. - ppt download

OpenStax College Physics for AP® Courses Solution, Chapter 29, Problem 11 (Test Prep for AP® Courses) | OpenStax College Physics Answers

.PNG)



.PNG)