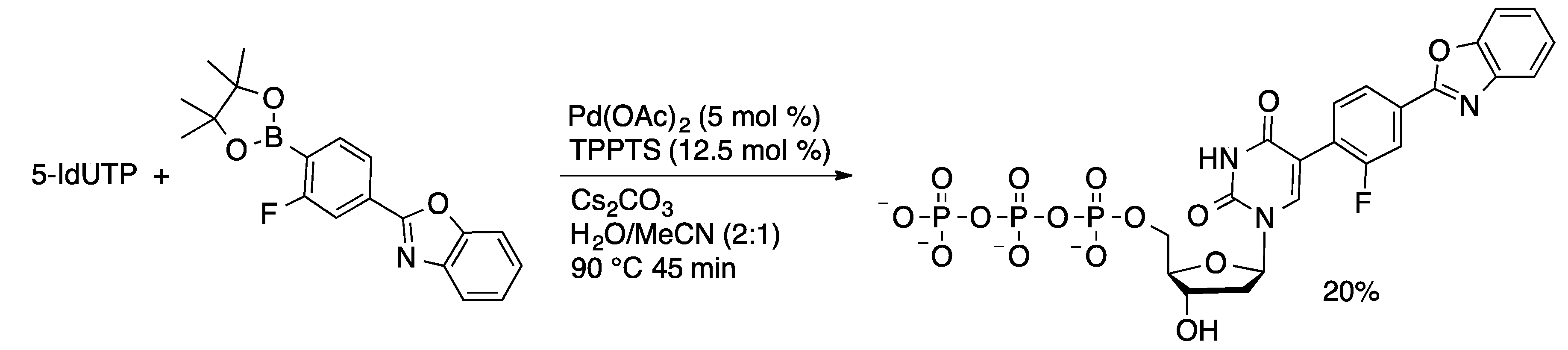
Molecules | Free Full-Text | Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides | HTML
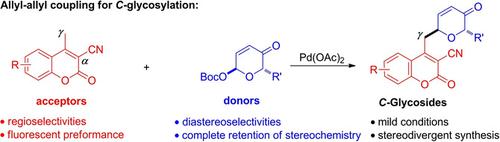
Palladium‐Catalyzed Regioselective and Diastereoselective C‐Glycosylation by Allyl‐Allyl Coupling,Advanced Synthesis & Catalysis - X-MOL

Suzuki Cross‐Coupling Reaction with Genetically Encoded Fluorosulfates for Fluorogenic Protein Labeling - Zhao - - Chemistry – A European Journal - Wiley Online Library
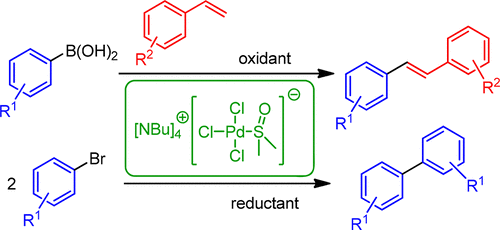
Oxidative and Reductive Cross-Coupling Reactions Catalyzed by an Anionic “Ligandless” Palladium Complex,Organic Process Research & Development - X-MOL

Catalysts | Free Full-Text | Rapid Sequentially Palladium Catalyzed Four-Component Synthesis of Novel Fluorescent Biaryl-Substituted Isoxazoles
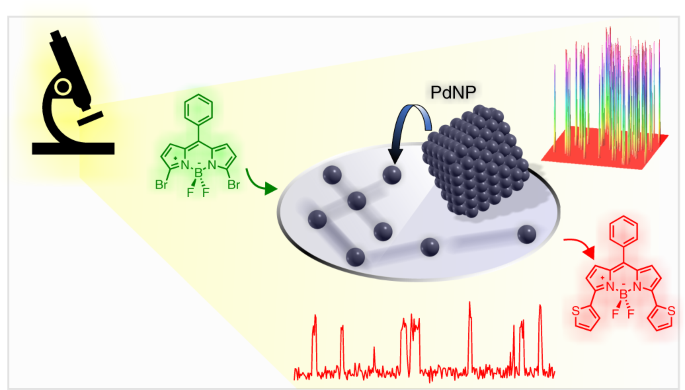
Real-time fluorescence imaging of a heterogeneously catalysed Suzuki–Miyaura reaction | Nature Catalysis

The Suzuki-Miyaura cross coupling catalyzed by palladium complexes 3a-e. | Download Scientific Diagram

Proposed mechanism for the palladium pincer-catalyzed carbonylative... | Download Scientific Diagram
![Synthesis of β‐Benzo[b]thienyldehydrophenylalanine Derivatives by One‐Pot Palladium‐Catalyzed Borylation and Suzuki Coupling (BSC) and Metal‐Assisted Intramolecular Cyclization – Studies of Fluorescence and Antimicrobial Activity - Abreu - 2005 ... Synthesis of β‐Benzo[b]thienyldehydrophenylalanine Derivatives by One‐Pot Palladium‐Catalyzed Borylation and Suzuki Coupling (BSC) and Metal‐Assisted Intramolecular Cyclization – Studies of Fluorescence and Antimicrobial Activity - Abreu - 2005 ...](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/2b5bc92b-a422-45aa-919a-1fdee01dc82e/mfig000.gif)
Synthesis of β‐Benzo[b]thienyldehydrophenylalanine Derivatives by One‐Pot Palladium‐Catalyzed Borylation and Suzuki Coupling (BSC) and Metal‐Assisted Intramolecular Cyclization – Studies of Fluorescence and Antimicrobial Activity - Abreu - 2005 ...
![Synthesis and Electroluminescence of Thiazolo[5,4-<em>b</em>]carbazole Based Blue Fluorescent Materials Synthesis and Electroluminescence of Thiazolo[5,4-<em>b</em>]carbazole Based Blue Fluorescent Materials](http://sioc-journal.cn/Jwk_yjhx/fileup/0253-2786/PIC/20191009173827.jpg)
Synthesis and Electroluminescence of Thiazolo[5,4-<em>b</em>]carbazole Based Blue Fluorescent Materials
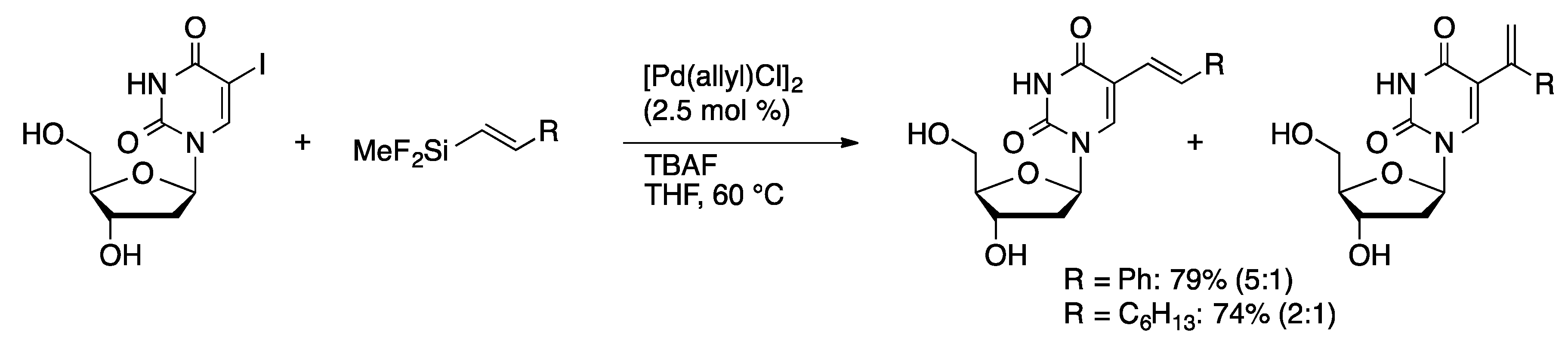
Molecules | Free Full-Text | Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides | HTML

3-Phenothiazinyl propiolates – Fluorescent electrophores by Sonogashira coupling of ethyl propiolate - ScienceDirect
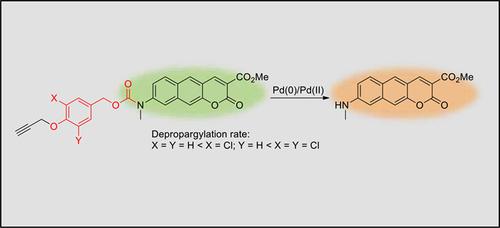
Electronic Effects on the Depropargylation Process in the Reaction‐based Fluorescent Detection of Palladium Species: Benzocoumarin‐based Ratiometric Sensing Systems,Bulletin of the Korean Chemical Society - X-MOL
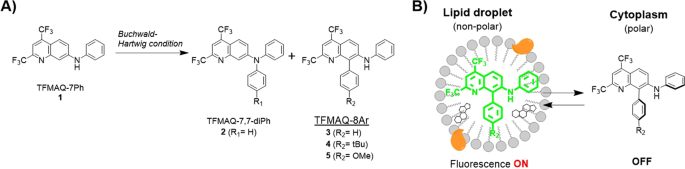
Selective synthesis of substituted amino-quinoline derivatives by C-H activation and fluorescence evaluation of their lipophilicity-responsive properties | Scientific Reports

A highly sensitive fluorescence method reveals the presence of palladium in a cross-coupling reaction mixture not treated with transition metals - ScienceDirect

New trends in the cross-coupling and other catalytic reactions in: Pure and Applied Chemistry Volume 89 Issue 10 (2017)
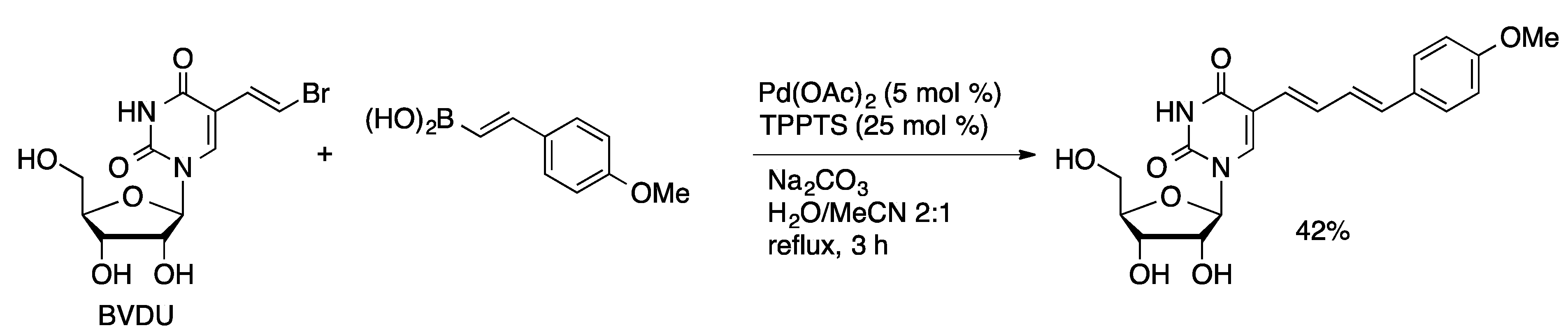
Molecules | Free Full-Text | Palladium-Catalyzed Modification of Unprotected Nucleosides, Nucleotides, and Oligonucleotides | HTML

Nanomolar Detection of Palladium (II) through a Novel Seleno-Rhodamine-based fluorescent and colorimetric chemosensor - ScienceDirect
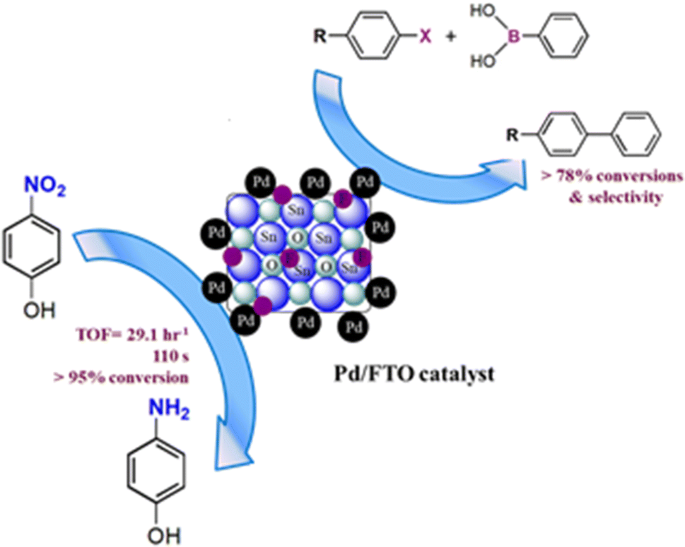
Palladium nanoparticles supported on fluorine-doped tin oxide as an efficient heterogeneous catalyst for Suzuki coupling and 4-nitrophenol reduction | SpringerLink
![Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo[e]indoles - ScienceDirect Palladium-catalyzed synthesis and fluorescence study of 2,3-diaryl-5-ethynylbenzo[e]indoles - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0040402017303848-fx1.jpg)

