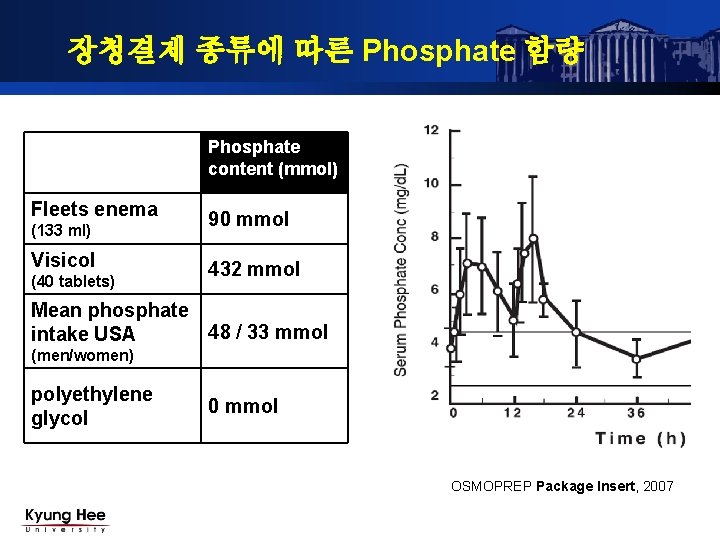
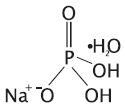
Sodium phosphate products available in the US: OsmoPrep and Visicol.... | Download Scientific Diagram

Medications Such As: Visocol, OsmoPrep, or Fleet Phoso-Soda May Cause Kidney Failure Or Death In The Elderly - Nursing Home Law Center

References in Residue-free sodium phosphate tablets (OsmoPrep) versus Visicol for colon cleansing: a randomized, investigator-blinded trial - Gastrointestinal Endoscopy
Osmoprep (sodium phosphate monobasic monohydrate, USP and sodium phosphate dibasic anhydrous, USP) Tablets and Visicol (sodium p